scpmkj@gmail.com
Get A Free Quote
Today, I would like to share with you some key points that need to be considered for assigning codes.
First: Choose the right supplier
Overall, assigning codes is an important stage in the implementation of UDI. If the selected supplier of coding equipment can also provide a mature supporting UDI software system platform, it will save a lot of time and energy costs during the implementation process. At the same time, it is also more conducive to optimizing procurement costs, production costs, personnel management costs, and time costs, making sales decisions and product processes clearer and clearer, and realizing visual management throughout the life cycle.
Second: Choose the right UDI label printer
As the carrier of UDI coding, the label needs to be attached to the product packaging. For different packaging materials, the corresponding coding equipment needs to be selected to ensure clarity and readability. Currently common UDI coding equipment are desktop thermal transfer printer, online thermal transfer TTO, UV inkjet printer, laser printer and thermal foam inkjet printer.
It is worth noting that the accuracy of the correspondence between packaging materials and coding machines does not mean that the label is in compliance. For example, the material of paper packaging boxes can generally be printed with a printer and then pasted with codes. However, since some local drug supervision or hospitals have specific requirements for coding methods, they can only use the specified methods to carry out coding. In this case, if packaging materials and coding methods are blindly selected without clear requirements, non-compliance may also occur.
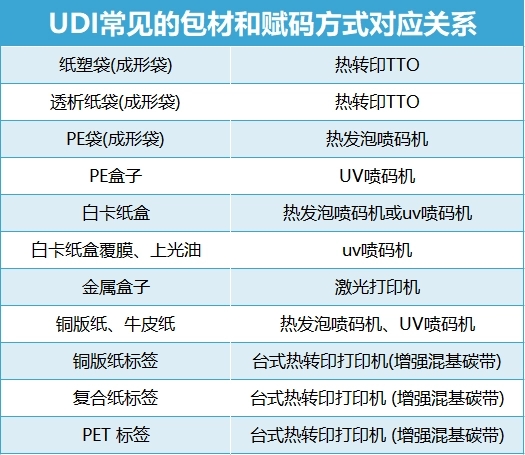
Third: Choose the UDI coding scheme that suits you
Among them, since there are two types of coding methods: mobile and mobile, and there are also many combinations of coding equipment, companies need to comprehensively consider the production conditions of existing products.
At present, there are generally two types of code assignment: manual and automated:
Mobile code assignment only requires the purchase of relatively simple relevant code assignment equipment. Generally, a computer + printer + scanning gun installed with the UDI service platform can successfully achieve compliant code assignment. This solution is suitable for enterprises with relatively high output and low degree of automation.
Automation requires the renovation and upgrading of the production line, which is suitable for medium and large enterprises with many product types and large output. This situation requires a higher degree of automation and requires the configuration of semi-automated production lines or automated production lines; At the same time, the UDI service platform is used to sort out and send data at the production line end, as well as collect data for the entire product life cycle, including multi-level packaging association (level 1, 2, 3, and 4 product packaging data association), so as to make it more convenient for inbound and outbound management and product life cycle management.
Fourth: Design of UDI labels
Before UDI labels are officially printed and assigned codes, they need to be designed and typesetting according to compliance standards. Generally speaking, UDI label design must include the following principles:
(1) If RFID is used, it must also include a one-dimensional barcode or a one-dimensional code label;
(2) The label cannot have only a code map, and needs to be accompanied by a logo description;
(3) The label printing quality complies with ISO15415 standard and above Level C;
(4) The UDI code and code map scanning content must be consistent and correct;
(5) Comply with AIDC and HRI principles (including machine readable part and human readable part)
Fifth: Label testing
Label testing is the last guarantee of compliance. The only labeling quality standard for medical devices forms a system, which is higher than ordinary reading standards. Therefore, clear printing of the UDI code and smooth scanning do not ensure that the label is of qualified quality.
In order to achieve label qualification, the following three items need to be done in place.
The first printing equipment must be selected correctly;
The printing template must be set correctly;
The third UDI conducts quality inspection and performs grade scoring:
In addition to meeting the relevant quality requirements for labels, UDI carriers should also be tested according to the following standards, and the barcode level of the inspection results should be above Level C.
One-dimensional code: GB/T 14258-2003 Information technology-Automatic identification and data acquisition technology-Inspection of the printing quality of bar code symbols.
dimensional code: GB/T 23704-2017 Inspection of printing quality of
In addition to the above five factors that need to be considered, selecting a one-dimensional code or a three-dimensional code, and whether the label position is accurate are also matters that need to be considered during the UDI code assignment process.
The above are some of the key points that companies need to pay attention to in the process of achieving compliance and coding for medical device products. If you have anything else you want to know, please feel free to consult us.






