scpmkj@gmail.com
Get A Free Quote
The unique medical device identification database is an important part of the unique medical device identification system. In accordance with the requirements of the "Rules for the Unique Medical Device Identification System" and the arrangements of the "Unique Medical Device Identification System Pilot Work Plan", the Unique Medical Device Identification Database was officially launched on December 10, 2019, opening unique identification for pilot varieties to pilot enterprises. Related data declaration function.
1. Build unique identification master data
The unique medical device identification consists of a product identification and a production identification. The unique medical device identification database will collect the product identification and related data of the unique medical device identification. The medical device registrant/filing person is responsible for the data declaration and responsible for the authenticity and accuracy of the data. Responsible for uniqueness. The unique identification database will provide master data of medical devices for all aspects of production, circulation and use, achieving the same origin of data and unified standards.
2. Promote the linkage of the entire chain
In accordance with the requirements of the "Reform Plan for the Governance of High-Value Medical Consumables" to "explore the connection and application of standardized coding in the registration, procurement, and use of high-value medical consumables", medical insurance coding fields and the classification of consumables and equipment have been added to the unique medical device identification database to promote the entire chain linkage of medical devices from source production to clinical use, realize multi-party data sharing, and promote the "three medical linkage".
3. Provide multiple service models
The unique medical device identification database is built on the principles of standardization, ease of use, openness and extensibility. The page design is simple and friendly, and it is convenient to operate. On the basis of fully considering user needs, it provides data declaration methods such as web declaration, template import and interface docking, as well as data sharing methods such as web online query, data batch download and interface docking, making it convenient for all types of users to choose their own declarations according to actual needs., sharing methods to provide users with a good experience.
4. Open a unique identification system column
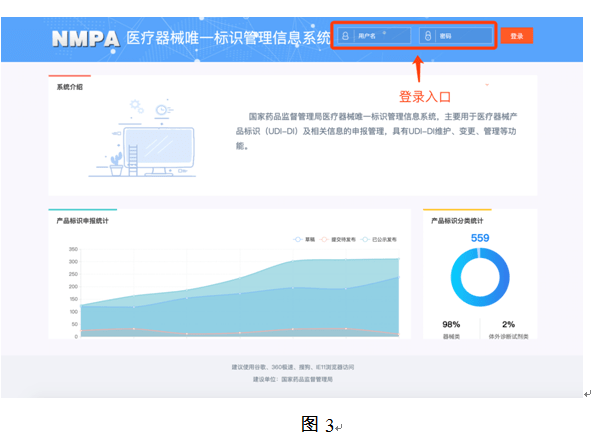
In order to facilitate the public to view the construction progress of the unique medical device identification system, the "Medical Devices" section of the State Food and Drug Administration website has opened a "Unique Medical Device Identification System" column (as shown in Figure 1). Click to enter to inquire about relevant policies, regulations, work trends, database operation guidelines, etc.(as shown in Figure 2), you can also access the unique identification database application login page (as shown in Figure 3) through the "Declaration Entrance" of this page.
In the next step, the State Food and Drug Administration will strengthen guidance and services for the application of unique identification data for medical devices, and open unique identification database query and sharing services to pilot units in March 2020 in accordance with the pilot work arrangement.
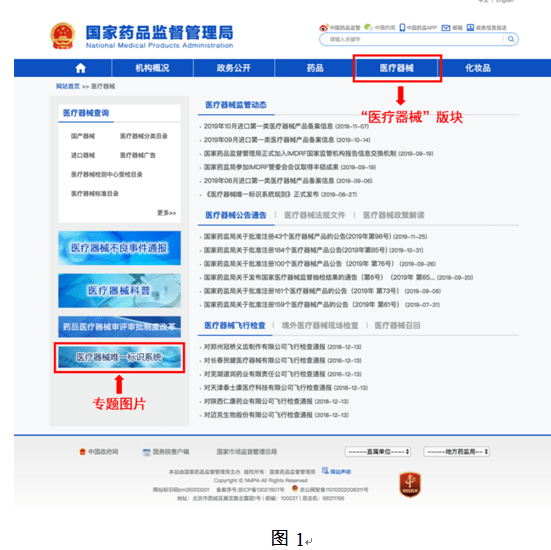
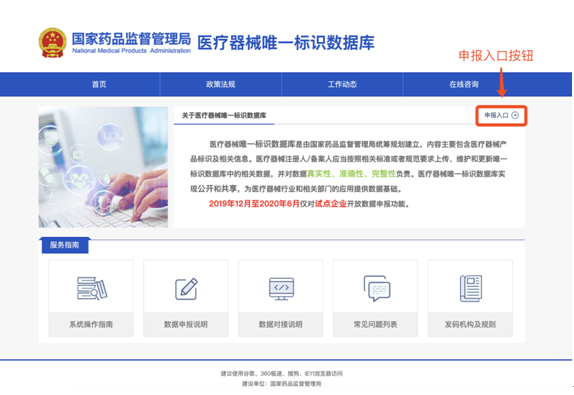
Fig. 2