scpmkj@gmail.com
Get A Free Quote
Pharmaceutical products are highly regulated commodities. In sales/use packaging at all levels, identification and coding play a vital role in ensuring consumer safety and brand protection. Without clear and easy-to-read information and data, compliance with laws and regulations and user safety cannot be guaranteed, and supply chain traceability costs will inevitably occur.

In the past 2021, many well-known pharmaceutical giants in North America alone (data from www.fda.gov) have recalled in batches their products sold outside, including missing product information, wrong specification identification, wrong dosage identification, information cannot be read, data cannot be traced, and a series of problems.

But how can we ensure the correctness of product coding and reduce the cost of unexpected losses without affecting the efficiency of the production line? There is a principle we can refer to: the sooner a problem is discovered, the less impact it will have. At the same time, there are at least a few aspects that should be taken into account:
01 Standard specifications of product packaging itself
Producing packaged products in only one or more size may face different technical options. Sometimes, the difference between them will be very large. From the perspective of coding technology, if ink coding is carried out with high resolution, the printing distance will be very close. If laser technology is used, whether a zoom function is needed due to changes in the printing surface. Or the transmission system can be compatible with adjustments of various specifications.
02 Information content to be marked on product packaging

This is closely related to the design of drug packaging. Whether to print only three-phase or drug traceability codes, one-dimensional barcodes or GS1 GTIN codes that need to contain two-dimensional codes, etc. What is the typesetting design of the information, the size arrangement of the characters, the use of coding rules for the QR code and how the matrix size is considered (although the information is the same, using different coding rules will affect the size of the QR code). What are the compliance requirements for coded information? Do you export abroad and consider the different rules and requirements of FDA, FMD, KNIH, SDC, DGFT, ANVISA, ITS in various countries?
03 Use of product packaging materials
Even if it is a paper outer box, whether coated or not coated will directly affect the effect of the coding technology. Due to the good permeability of uncoated cartons, alcohol or water-based ink jet printing can be considered, while coated cartons need to consider quick-drying type solvent-based ink for operation. It may also be applied by laser burning technology, but the impact on the contrast effect produced by different materials after burning may be great. This will increase the difficulty of identifying subsequent verification segments. Moreover, different coding technologies are accompanied by different printing speeds and are associated with different coding qualities. It should be considered clearly whether they can meet customer needs.
04 Requirements for visual verification/inspection
Potential faults and problems always exist, and the ability to detect problems as soon as possible after they occur is a key node in the code assignment inspection of pharmaceutical packaging. The earlier the problem is discovered, the complexity and even the loss of processing will be greatly reduced. Then, after the coding process of pharmaceutical product packaging, the importance of following up with visual inspection is self-evident.
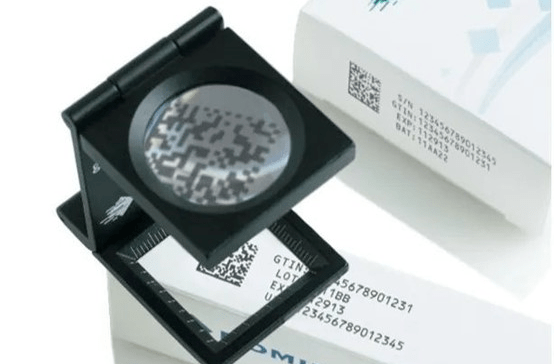
But the question arises. For visual inspection, what conditions do we test? Is there any code assigned? Missing partial code assignment? Can bar codes/QR codes be read? These are just the basics. Next, let's consider that QR codes can be read, but what is its level? If it is incorporated into subsequent sections or may be worn/attenuated during transportation (the fading speed of ink light), light reading may not be enough. Is the requirement for code assignment level detection also required? It's not over yet. Do you consider whether the content verification of all characters except bar codes/QR codes can be read? Are the contents that should be correctly coded after reading and planned consistent? This is the result we want to achieve, but the realization at the technical level also needs to consider the characteristics of different coding equipment, such as scribe laser, high-resolution thermal foaming, dot matrix continuous inkjet, print-quality thermal transfer and so on. They will produce different image characteristics and character edge types, and even present different "fault" phenomena when a code assignment failure occurs. For the visual verification process section, this involves the recognition image learning process in different situations and requires a certain amount of personnel technical experience and time to improve.
05 Considerations for data association
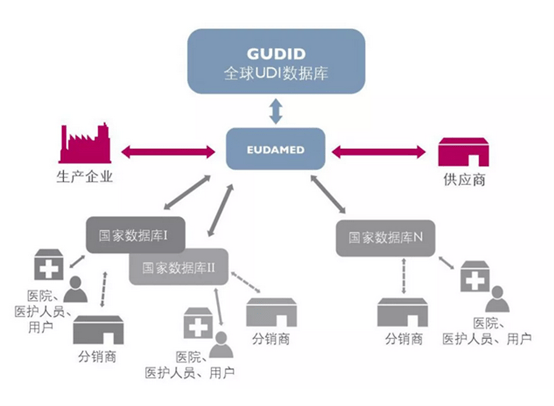
To put it simply, from code assignment to visual detection, the input and output of data are related to the entire system. If it is a one-item-one-code identification requirement, whether the data at the current packaging level still needs to be correlated? From single item to middle package to outer box stack and pallet, this series of data associations need to be considered, and from the beginning, it is necessary to ensure a high degree of accuracy from the beginning of package code assignment testing.
06 Operation process for defective products

Without 100% OEE (Global Comprehensive Production Efficiency), there will always be various problems leading to losses and defective products. In the process of packaging code assignment, when such a situation occurs, we can detect it in time, and it is very important to be able to deal with it as soon as possible. If there are more levels of association in the future, it will be troublesome once the queue is confused. The operators on the scene will be confused and will desperately search for defective products and even waste a large part of the product. Accurate, rapid, and correct corresponding elimination should be taken into account, and at the same time, data feedback of coded defective products and image storage or uploading for visual inspection should be improved.
07 Compliance certification

Unlike other products, pharmaceutical manufacturing has extremely strict compliance requirements. From equipment operation to digital signatures, from the composition of coded inks to GMP production, every flaw may affect the overall compliance of pharmaceutical companies 'products. Does the code assignment detection function used meet/meet 21 CFR part11, ePedigree? Do the identification ink products used also comply with GMP production standards? It also requires careful consideration.
Domino Pharmaceutical packaging coding and testing solution fully considers the above seven points, which can help companies ensure the correctness of product coding and greatly reduce production line costs. Domino has more than 40 years of experience in solutions in pharmaceutical product traceability, GMP compliance and combating counterfeit drugs. We are experts in "standard" and "knowledge" and can provide users with one-stop solutions.

One-stop solution for visual inspection of Domino outer box code assignment
Domino Printing Vision Workstation (CV Station), used for serialization information of box packaging, including printing various barcodes, QR codes, batch numbers, product identification numbers (GS1\GTIN), expiration dates, pattern logos, etc., and also includes a visual inspection system that can meet functions such as MRC and ORC and defective product elimination plans.
There is no need to spend extra energy to coordinate the integration of various equipment manufacturers. This is a mature and efficient external box code assignment solution. After understanding the reserved stations on the production line, we will customize the printing vision workstation that matches the line size and seamlessly integrate it into the line station you plan.
1 Serialized information printing
One-dimensional code, two-dimensional code, batch number, expiration date and product identification number (e.g.: GS1 GTIN)
Comply with ISO/IEC 15415 barcode level testing
Production speed of up to 400 boxes per minute
Automatic saving of serial number and lot number data
2 Quickly integrate into your production line
Develop suitable process parameters based on your production line conditions
Production line speeds of up to 60 meters/minute
Synchronous upper and lower clamping conveyor belt design for stability and accuracy
Compatible with both permeable and non-permeable packaging materials
High-speed pneumatic removal of defective products
3 Strong visual inspection capabilities

Inspection of drug coding content
MRC and ORC identification testing
Save or upload defective product detection images
Online Bar Code Level Inspection (ABCDF)(optional)
21 CFR Part 11 compliance






