scpmkj@gmail.com
Get A Free Quote
Medication errors are one of the leading causes of avoidable harm in global health care.
Recognizing the benefits of product data in helping protect patient health and safety, major stakeholders, including hospitals, pharmacies and health care providers, are putting pressure on drugmakers to go further.
Reduce medication errors
A simple medication error can have devastating effects, leading to serious patient injury, disability and even death.
A stricter serialization measure-the identification of single doses of drugs-can help reduce unsafe medication and medication errors, and ensure that patients receive the right dose of the right drug at the right time.
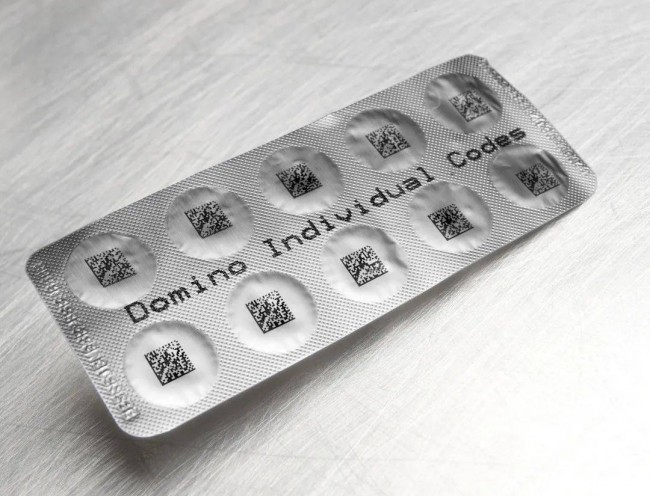
For example, adding more detailed data to strips and blister packages could help hospitals, nursing homes and other medical institutions better control drug use. Scanning the serialized QR code automatically verifies the medication to ensure that patients receive the correct medication and dose. This level of refinement reduces allocation errors and improves inventory management.
Single dose identification
Nowadays, the secondary packaging of products is usually assigned a QR code, which contains information such as the product, dose, batch code and expiration date. The next level can be assigned to single doses of each product, which may include vials, ampoules, and individual blister bags containing tablets.
The benefits of single-dose serialization are obvious, but it takes the collaborative efforts of pharmacies, doctors, hospitals and other health care providers to drive change. To welcome the era of single-dose serialization, pharmaceutical manufacturers may need to update their current coding and identification functions.
While drugmakers can now ensure that serialized QR codes are printed correctly for effective scanning, single-dose coding poses additional challenges.

When assigning products online, product processing is the basis for achieving high-quality coding, especially for manufacturers who want to explore single-dose labeling.
Factors that affect the quality of final code assignment include:
● Product location: Minor changes in the product location on the production line may cause the assigned code to be applied to the wrong area or the assigned code to be missing.
● Distance between product and coding equipment: Too close or too far away from the coding equipment may cause the coding to be ambiguous or unreadable.
● Product angle: A slight rotation of product positioning, even a few degrees, may cause code assignment deformation.
● Linear speed: Speed fluctuations may cause code compression or stretching.
● Conveyor belt vibration: At high speeds, vibration may cause low code quality, blurring or wavy shapes.
● Challenging geometry products: Certain packaging types may be challenging for standard coding settings.

Low-quality QR codes caused by improper product handling can cause scrap, rework and inventory defects. If unreadable QR codes enter the market, the consequences will be even more serious. Introducing single-dose labeling may increase this risk and complexity.
data needs
While introducing single-dose identification can enhance traceability and help reduce errors, the amount of data generated, tracked and managed can present challenges for manufacturers and supply chains.
For example, suppose a blister pack contains seven tablets and produces one million packs. Typically, manufacturers must create, store and print one million assigned codes to meet the serialization requirements for each package. The data requirement to track individual blister bags has increased to 7 million.
Globally, the regulatory requirements for serialization are becoming more complete and strict, and manufacturers are the key to implementing solutions. Cost is no longer an obstacle and manufacturers can obtain more advanced labeling and serialization systems at lower prices, but again, the implementation of any new technology will pose challenges and manufacturers need to strike the right balance and work with the right suppliers to gain a competitive advantage.
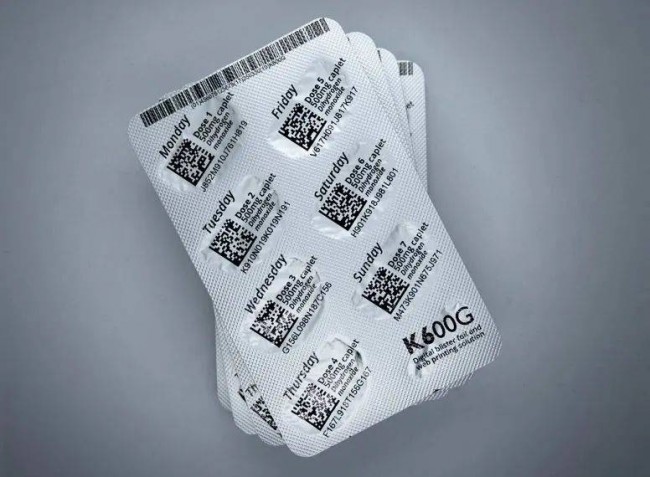
Domino K600G innovative digital printing solution for aluminum foil blisters
Adopting online digital printing solutions provides necessary flexibility and allows manufacturers to respond more quickly to changes in printing requirements. For drug manufacturers, one of the significant benefits of using the K600G digital printing solution is that single-dose serialization requirements can be met, and unique codes and serial numbers can be printed on every pocket of a blister package.
Patient safety is a key driver of reform in the pharmaceutical industry, and the need for higher levels of traceability and accountability is bound to increase, so now is the time to prepare.






